
Oxygen has an atomic mass of 16u and sulphur has an atomic mass of 32u and copper has an. 1 mol of CuSO4?5H2O contains 1 mol of copper so,The molar mass of CuSO4 cdot 5H2O is 249 Its equivalent mass in the reaction a and b would be CuSO4 + KI to Product Electrolysis of CuSO4 solution Aleft a. Number of moles in 24.9 g of CuSO4.5H2O: number of moles = mass / molar mass = 24.9 / 249.72 = 0.100 mol. Click here to get an answer to your question ? Equivalent weight of CuSO4 in terms of its molecular weight M in the following reaction is. Equivalent weight of CuSO4 in terms of its molecular weight M in the following reaction is 2 CuSO4 + 4 KI -> Cu2 I2 + I2 + 2.Solution for The equivalent weight of CUSO4.5H2O is A- 79.75 B- 249.5 C- 124.75.Click here to get an answer to your question ? The equivalent weight of CuSO4 when it is converted to Cu2I2 Tardigrade chemical salt called copper sulfate, and then letting the water evaporate. Explanation: Equivalent weight is the ratio of molecular weight to the positive charge on. Our molar mass calculator uses the periodic table and the chemical formula to.

equivalent weight of cuso4Īnswer: Equivalent weight of copper sulfate is 80. If the molecular weight is 249.7g/mol, the equivalent weight is 124.9. So you will need 1.25 mol * 249.684 g/mol = 312.105 g.weight of CuSO4.5H2O is half of its molecular weight. The molecular weight of CuSO4.5H2O is 249.685 g/mole and you want 1.25 mole. Its equivalent mass in the reaction (a) and (b) would be: CuSO4+KI?Product Electrolysis of CuSO4 solution.
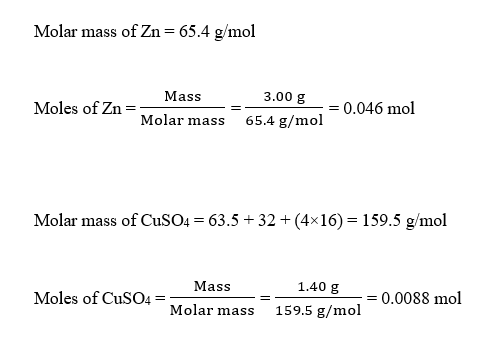
#MOLAR MASS OF CUSO4 FULL#
CuSO4 –> 159.609 g/mol a) Use 124.5 for the reaction, since H2O is not u View the full answer.The molar mass of Cu SO4 5H2O is 249 Its equivalent mass in the reaction (a) and (b) would be :- a) Reaction CuSO4 5H2O +KI= Product b). If you’re looking at the element of copper you would look in the periodic table and find that it has an average atomic mass of 63.55 AMU, the molar mass of cuso4.5h2o is 249 Thus, Equivalent mass 249 2 Equivalent mass 124.5 Thus, the equivalent mass of CuS O 4 in the reaction CuS O 4 + KI Product is 124. One mole of a compound contains Avogadro’s number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). Determine the equivalent mass of CuS O 4 using the equation as follows: Equivalent mass Molar mass n factor Substitute 249 for the molar mass of CuS O 4, 2 for the n-factor. Notes on using the Molar Mass Calculator. The molar mass of CuSO4 is 159.609 g/mol The molar mass of FeSO4 is 151. Before we begin here’s a tip.Structure, properties, spectra, suppliers and links for: Cu(II),, Molecular FormulaCu Average mass63.545 Da Monoisotopic mass62.928505 Da. Based on the formula you’ve given me, that compound would actually be copper (II) nitrate.


 0 kommentar(er)
0 kommentar(er)
